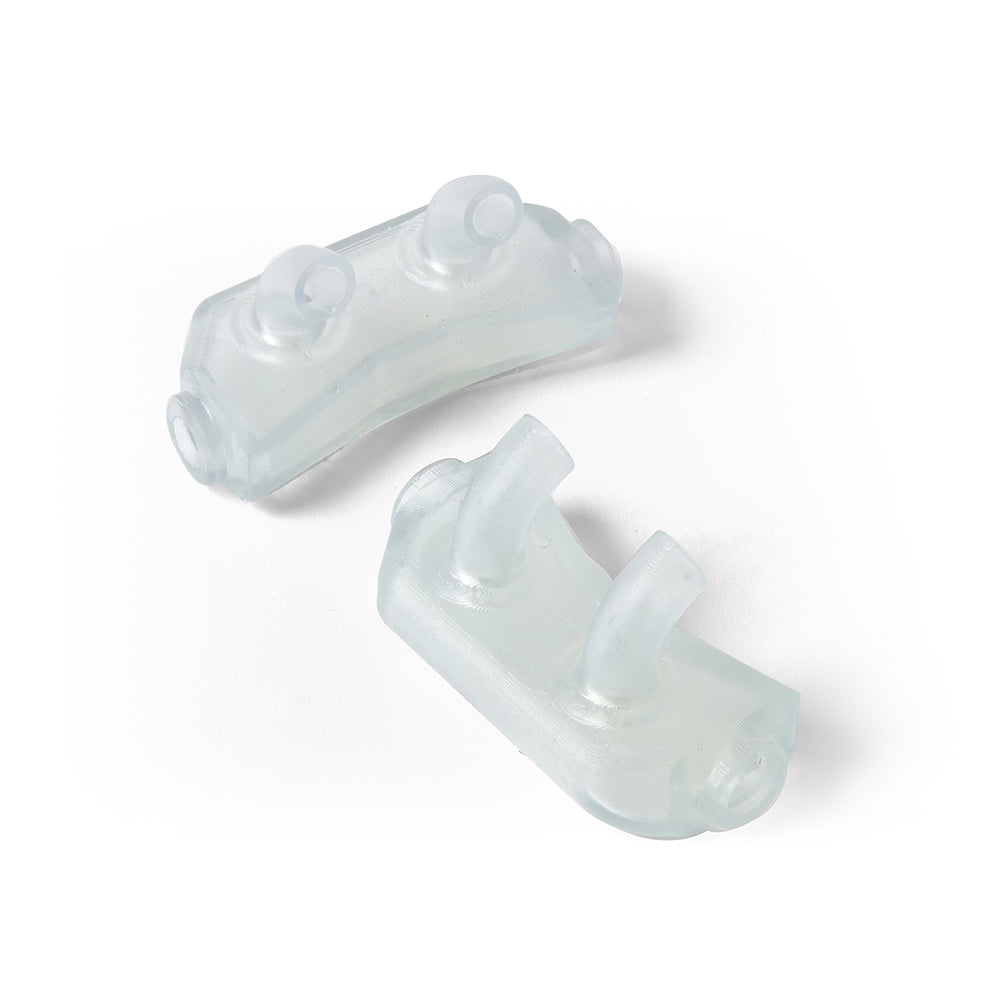
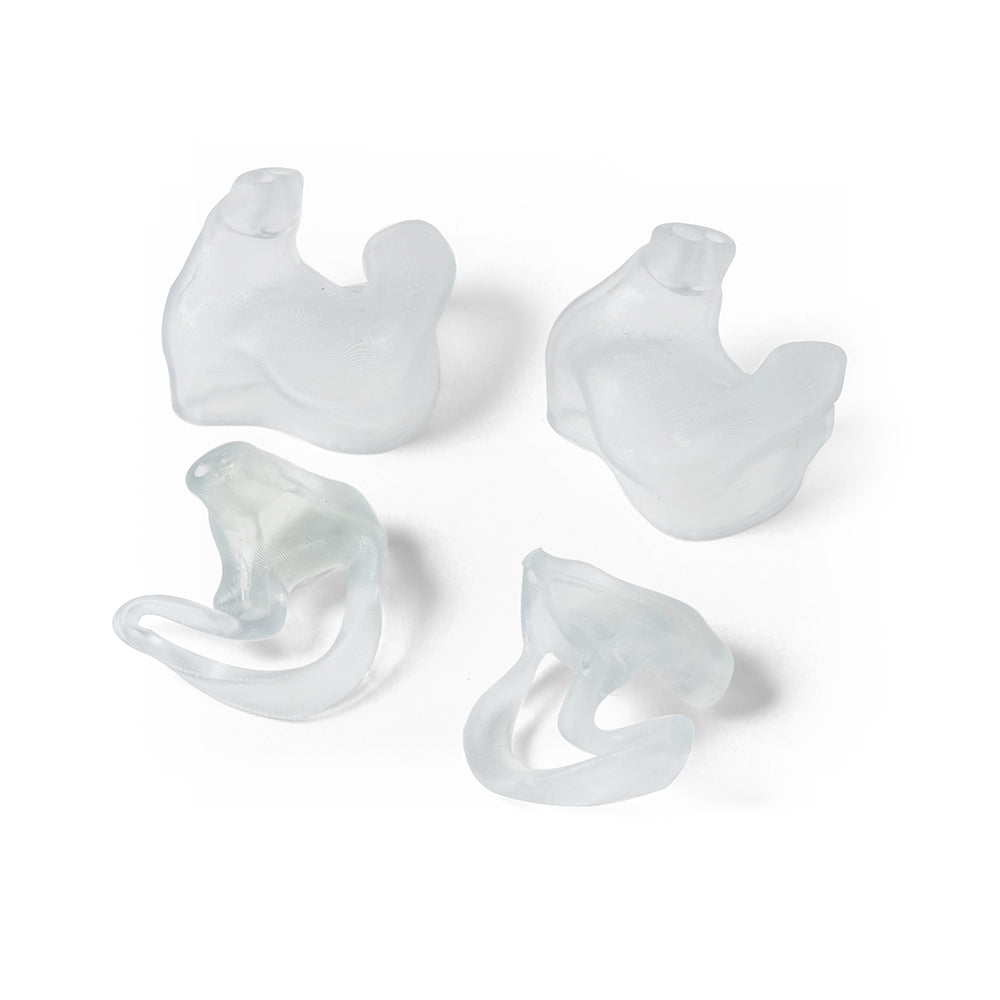
BioMed Flex 80A Resin is a firm, flexible, medical-grade material for applications requiring durability, biocompatibility, and transparency. This ISO 10993 and USP Class VI certified material is made in an FDA-registered, ISO 13485 facility and can be used in applications for long-term skin (>30 days), and short-term mucosal membrane contact (less than 24 hours).
BioMed Flex 80A is the firmest flexible material in our biocompatible family of BioMed resins, empowering healthcare professionals and medical device engineers with new design possibilities and efficiencies. Customers can choose BioMed Flex 80A Resin to directly print patient-specific medical devices requiring flexibility or firm tissue models surgeons can reference in the O.R. By directly printing components, BioMed Flex 80A Resin eliminates labor time and cost.
Equipped with a shore hardness of 80A and 120% elongation at break, this ISO 10993 and USP Class VI certified material is produced in an FDA-registered, ISO 13485 facility and can be used in applications requiring long-term skin contact or short-term mucosal membrane contact.
Directly 3D print flexible parts requiring biocompatibility with BioMed Flex 80A Resin. Reduce workflow times by eliminating molding to directly produce flexible, patient-specific medical devices or firm tissue medical models surgeons can reference in the O.R.
BioMed Flex 80A Resin is ideal for:
Achieve new design possibilities and efficiencies using 3D printing and BioMed Flex 80A Resin, from producing flexible biocompatible medical devices to anatomical models requiring biocompatibility.
Flexible
Produce flexible medical devices or firm tissue models requiring short-term mucosal membrane contact that can optimize mass personalization applications versus using cumbersome molds.
Transparent
Produce complex transparent firm tissue models and bring them directly into the operating room (O.R.) as a reference for complex medical procedures (eg. sizing models, cartilage models, or disease organs.)
Medical-Grade Material, Biocompatible Parts
Leverage a material manufactured within our Quality Management Systems, with strict adherence to ISO 13485. Instill confidence that this material is suitable for biocompatible applications with long-term skin (>30 days), short-term mucosal membrane contact (<24 hours), and USP Class VI certifications.

Request a Free Custom Quote
Use our easy tool to build a customized quote.
Request a Custom 3D Print Sample
Let us prove that the technology will work for your use case.
Speak with an Expert
Talk 1:1 with an expert. Tell us your challenges and our team will help you find the best solutions to meet your unique needs.